Table 1 Optimization for the reaction condition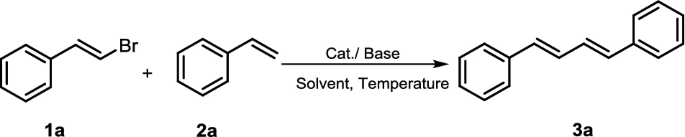

From: Synthesis of (1E,3E)-1,4-diarylbuta-1,3-dienes promoted by μ-OMs palladium–dimer complex
Entry | Catalyst/5% | Solvent | Base | T/°C | t/h | Yield/%a |
|---|---|---|---|---|---|---|
1 | Pd(OAc)2 | Toluene | K2CO3 | 80 | 36 | 19 |
2 | Pd2(dba)3 | Toluene | K2CO3 | 80 | 24 | 11 |
3 | Pd(OAcCF3)2 | Toluene | K2CO3 | 80 | 24 | 15 |
4 | μ-OMs dimer | Toluene | K2CO3 | 80 | 36 | 51 |
5b | μ-OMs dimer/PPh3 | Toluene | K2CO3 | 80 | 24 | Trace |
6 | Pd-Xphos | Toluene | K2CO3 | 80 | 24 | Mix |
7 | μ-OMs dimer | Toluene | K2CO3 | 120 | 18 | 74 |
8 | μ-OMs dimer | DMF | K2CO3 | 120 | 12 | 52 |
9 | μ-OMs dimer | CH3CN | K2CO3 | 120 | 12 | 21 |
10 | μ-OMs dimer | NMP | K2CO3 | 120 | 12 | 50 |
11 | μ-OMs dimer | DMSO | K2CO3 | 120 | 12 | 48 |
12 | μ-OMs dimer | Toluene | Li2CO3 | 120 | 24 | mix |
13 | μ-OMs dimer | Toluene | Cs2CO3 | 120 | 15 | 68 |
14 | μ-OMs dimer | Toluene | K3PO4 | 120 | 20 | 62 |
15 | μ-OMs dimer | Toluene | Et3N | 120 | 18 | 70 |
16c | μ-OMs dimer | Toluene | K2CO3 | 120 | 24 | 65 |