Table 1 Tyrosinase inhibitory effects of synthesized compounds 3a–j in comparison with kojic acid and Binding energy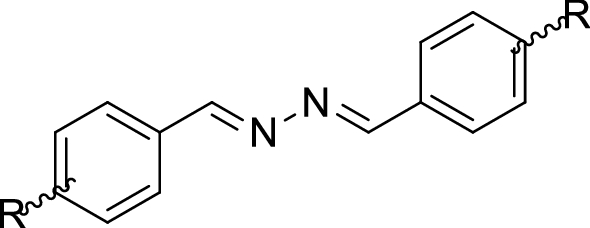

Tyrosinase inhibitory activitya | ||
|---|---|---|
Compounds | R | IC50 (µM)b |
3a | H | > 100 |
3b | 2-OMe | > 100 |
3c | 4-OMe | > 100 |
3d | 3-OEt-4-OH | > 100 |
3e | 3,4,5-(OMe)3 | 20.10 ± 0.01 |
3f | 2,4-(OH)2 | 7.30 ± 1.15 |
3g | 3,4-(OMe)2 | > 100 |
3h | 3-OH-4-OMe | 57.34 ± 0.02 |
3i | 4-(OCOCH3)-3-OMe | 28.11 ± 0.52 |
3j | 4-OH | 62.60 ± 0.71 |
3k | 4-OH-3-OMe | 12.90 ± 0.18 |
Kojic acid | – | 20.24 ± 2.28 |