Scheme 1
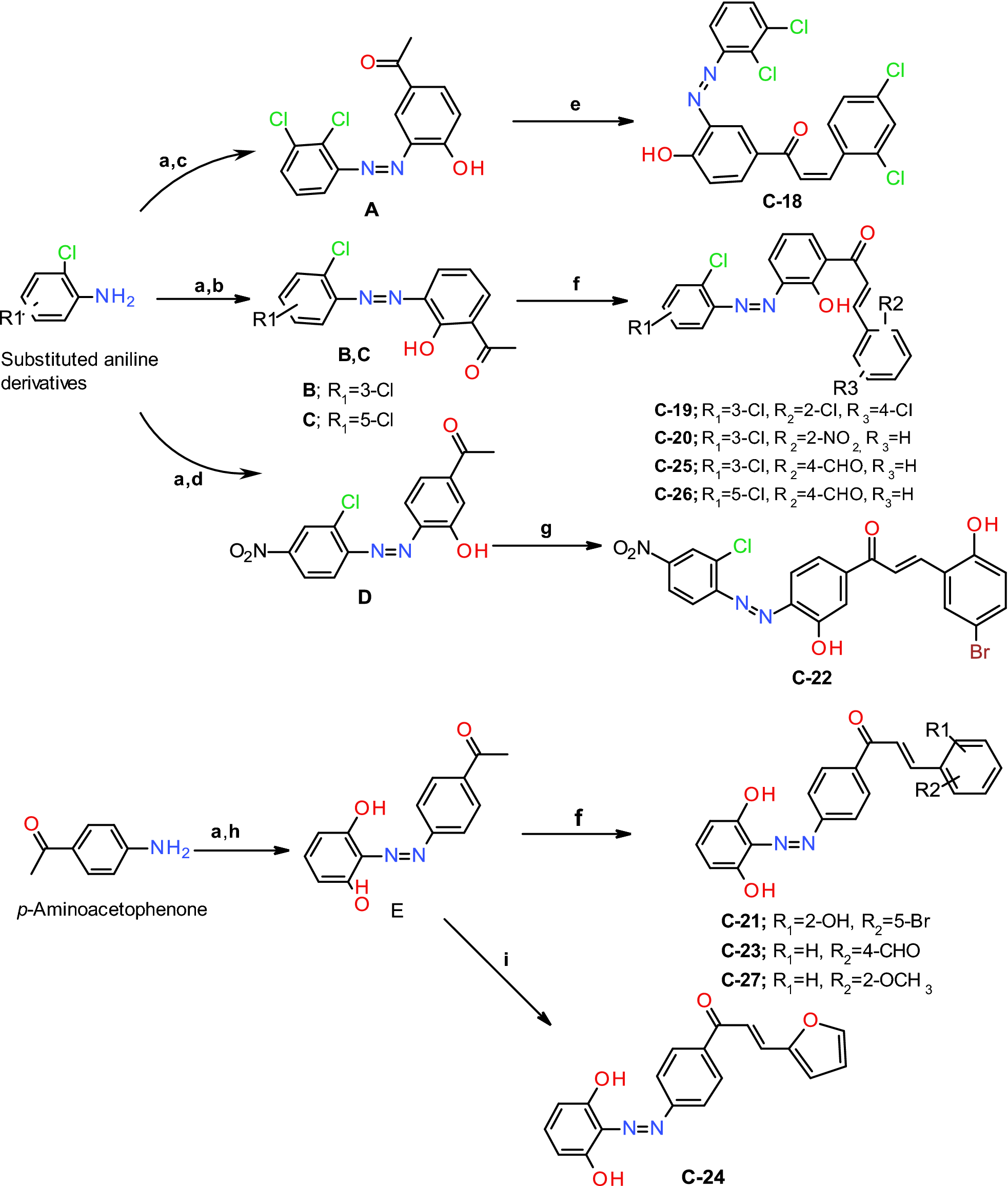
Synthesis of diazenyl chalcones (C18–C-27). Reaction and reagents: (a) sodium nitrite, HCl, 0 °C, (b) o-hydroxyacetophenone, ethanol, 10% NaOH; (c) p-hydroxyacetophenone, ethanol, 10% NaOH; (d) m-hydroxyacetophenone, ethanol, 10% NaOH; (e) 2,4 dichlorobenzaldehyde, ethanol, 7–8 drops of 50% NaOH, stirring for 16 h at rt; (f) different mono or di-substituted benzaldehydes, ethanol, 7–8 drops of 50% NaOH, stirring for 18–24 h at rt; (g) 5-bromosalicylaldehyde, ethanol, 7–8 drops of 50% NaOH, stirring for 18 h at rt; (h) resorcinol, ethanol, 10% NaOH; (i) furfural, ethanol, 7–8 drops of 50% NaOH, stirring for 20 h at rt