Table 2 Dehalogenation and regioselective demethoxylation of 1a–1c, 2a–2c and 3a–3c
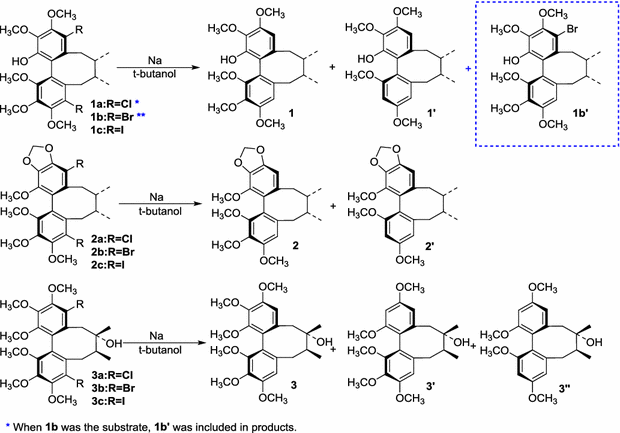

Entrya | Substrate | Products (Convb, %) | ||
|---|---|---|---|---|
1 | 1a | – | 1 (2) | 1′ (83) |
2 | 1b | 1b′ (5) | 1 (10) | 1′ (71) |
3 | 1c | – | 1 (35) | 1′ (53) |
4 | 2a | – | 2 (16) | 2′ (62) |
5 | 2b | – | 2 (26) | 2′ (57) |
6 | 2c | – | 2 (37) | 2′ (57) |
7 | 3a | 3 (20) | 3′ (12) | 3′′ (50) |
8 | 3b | 3 (23) | 3′ (18) | 3′′ (58) |
9 | 3c | 3 (24) | 3′ (14) | 3′ (62) |