Table 1 Optimization for reaction conditions of dehalogenation and regioselective demethoxylation of 4, 11-dichloroschisandhenol (1a)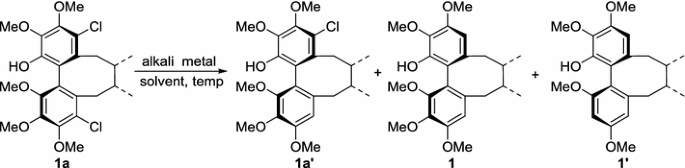

Entrya | Alkalimetal | Eq.b | Solvent | [C] (mM) | Temp (°C) | Time (h) | Products (convc, %) |
|---|---|---|---|---|---|---|---|
1 | Na | 100 | Absolute ethanol | 14 | rt | 2 | 1a′ (0); 1 (0); 1′ (47) |
2 | Na | 100 | t-Butanol | 9 | rt | 24 | 1a′ (0); 1 (0); 1′ (63) |
3 | K | 100 | t-Butanol | 14 | 50 | 3 | 1a′ (0); 1 (0); 1′ (40) |
4 | K | 30 | Dry THF | 5 | rt | 24 | 1a′ (0); 1 (1); 1′ (41) |
5 | Na | 100 | t-Butanol | 5 | 60 | < 4 | 1a′ (7); 1 (8); 1′ (56) |
6 | Na | 100 | t-Butanol | 5 | 40 | 12 | 1a′ (0); 1 (29); 1′ (0) |
7 | Na | 100 | t-Butanol | 5 | 50 | 4 | 1a′ (0); 1 (1); 1′ (83) |
8 | Na | 50 | t-Butanol | 5 | 50 | < 4 | 1a′ (1); 1 (2); 1′ (48) |
9 | Na | 75 | t-Butanol | 5 | 50 | < 4 | 1a′ (0); 1 (3); 1′ (61) |
10 | Na | 125 | t-Butanol | 5 | 50 | 12 | 1a′ (1); 1 (2); 1′ (86) |
11 | Na | 125 | t-Butanol | 4 | 50 | 5 | 1a′ (0); 1 (0); 1′ (53) |